A new ENT device: UniSpray
27/10/2021

Nemera launches UniSpray, its unidose system primarily for systemic-acting drug administration.
Nemera has a proven track record in developing and manufacturing complex Ear Nose and Throat solutions. In line with clear growing market interest in unidose system coupled with promising advantages that nasal route could offer, Nemera is thrilled to extend its portfolio by launching its unidose system, UniSpray, for systemic-acting drug administration to face emergency and crisis.
Other than treating conditions locally, nasal delivery is an attractive option to also administer systemic therapies which may vary from benign to serious health conditions. What’s more, is that the nasal route is not invasive and does not require healthcare professionals’ intervention where patients can self-administer their remedy with a rapid onset.
UniSpray is a ready-to-use primeless device with single-metered liquid dose delivery. It offers one-handed activation with 360° functionality to face emergency and crisis. Thanks to its ergonomic design, it is intuitive and easy-to-use.
UniSpray is a unidose system concept under development, designed to be robust and compatible with existing marketed primary containers. Through usability analysis and Freedom to Operate assessment, we assure reliability and regulatory compliance of our unidose concept.
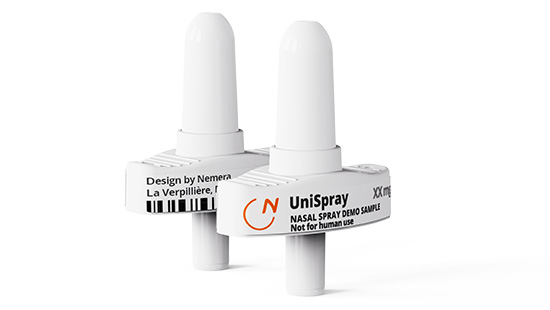
UniSpray at a glance:
BENEFITS
- Intuitive and robust device with ergonomic finger rest, and broad labeling surface
- Safe and secured with no risk of accidental activation
- Accelerates time-to-market thanks to possible adaptations to conventional filling lines
- Equivalent utilizationas originators In-vitro Bioequivalence services proposed with the device
- Ready-to-use, primeless device, 360° functionality
- Compatible with existing primary drug container
- Single dose liquid delivery device with One accurate 100µL dose
- Compliant to regulatory exigence
- Freedom to Operate (FTO)
- Customization possibilities
- For new and repurposed drug
To download the press release : HERE
About Nemera
As a world-leading drug delivery device solutions provider, our purpose of putting patients first enables us to design and manufacture devices that maximize treatment efficacy. We are a holistic partner and help our customers succeed in the sprint to market of their combination products. From early device strategy to state-of-the-art manufacturing, we’re committed to the highest quality standards. Agile and open-minded, we work with our customers as colleagues. Together, we go the extra mile to fulfill our mission.
For more information, visit www.nemera.net


