Seen in the press: Nemera’s article featured in February ‘Prefilled syringes & Injection Drug delivery’
05/06/2021

“Prefilled Syringes & Injection Drug delivery” magazine features Nemera’s article about the importance of device optimization to increase patient safety during self-administration of medication.
The recently published “Prefilled syringes & Injection Drug delivery” magazine includes an article from Nemera: “LEVERAGING THE PATIENT JOURNEY TO OPTIMISE DEVICE USE AT HOME”.
In this article, Séverine Duband, Global Category Manager, and Mark Tunkel, Director of Business Development, Insight Innovation Center, both at Nemera, discuss the importance of device optimization to increase patient safety during self-administration of medication.

The growing prevalence of chronic diseases, along with the evolution of patients’ lifestyles, is driving new ways of administrating parenteral drugs. Novel treatments have become available, with the majority arising from biological molecules. Biologic drugs are predominantly administered via injectable devices, using prefilled syringes as a base.
Thanks to the increased early-stage development capabilities offered by the recent integration of newly acquired capabilities with the Insight Innovation Center, the Nemera teams in Europe and the US can now be a single partner for device platforms and integrated services – from front-end innovation, design research, human factors, and design engineering to strong l
ate-stage development, as well as clinical and commercial manufacturing capabilities.
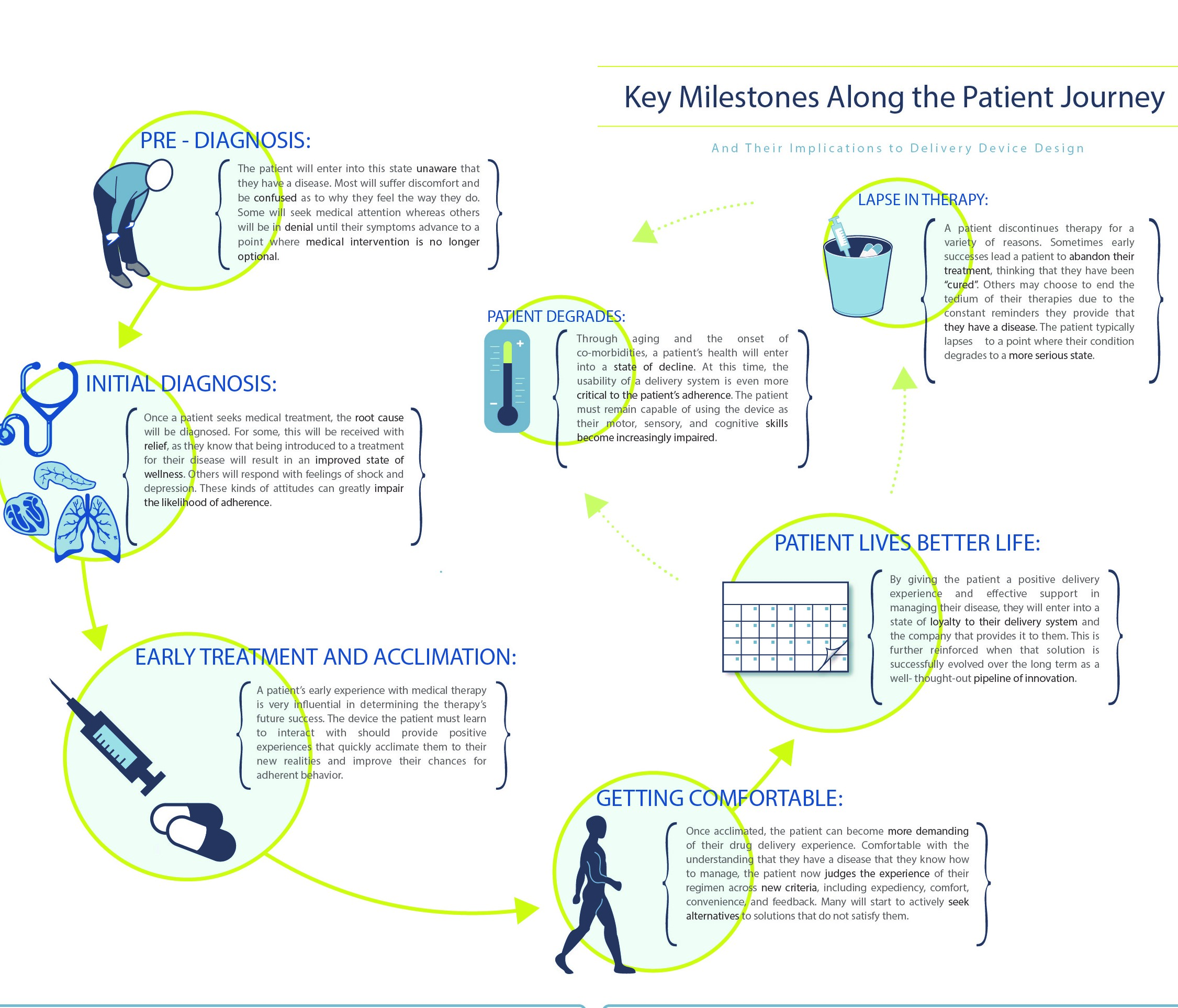
Understanding both the patient and healthcare provider experience enables the development of the patient journey and clinical environment process maps, which demonstrate the complete process patients go through in managing their disease – both from an administration standpoint and from a longitudinal perspective – as they progress with their condition and treatment.
Regulation and recommendations are evolving to improve patient and health worker safety. In this context, Nemera’s Safe’n’Sound® product range matches the need for safe and easy-to-use self-deliveries – this passive safety device for prefilled syringes provides increased safety for patients and healthcare professionals.
The full issue of the magazine:
Prefilled syringes & Injection devices Drug Delivery, Issue n.105, February 2020
About Nemera
Nemera is a world leader in the design, development, and manufacturing of drug delivery devices for the pharmaceutical, biotechnology and generics industries. Nemera offers a comprehensive portfolio of products and services across ophthalmology, nasal, inhalation, dermal, transdermal and parenteral delivery. Nemera’s vision is to be the most patient-centric drug delivery device company. Nemera always puts patients first, providing high-quality solutions that have a demonstrable impact on patients’ health
Nemera’s newly branded Insight Innovation Center, with offices in North America and Europe, provides consultative services to support your overall device strategy. Providing user research, Human Factors, User Experience design, and Design for manufacturing, the Insight Innovation Center can help customers navigate their device strategy for both novel and platform solutions. Users are at the center of everything that we do in our effort to always put patients first.


