Patient Journey as the foundation for holistic connected device development
22/02/2023

An Increasingly Complex Landscape for Connected Devices
The methods available to pharmaceutical companies and device suppliers to support the patient journey and experience are offering more possibilities than ever before. Traditionally, the focus of the patient experience has been on optimizing the “administration task” as outlined in the instructions for use, with an emphasis on the reduction of use error risk. However, as clinical outcomes are increasingly driving payer decision making, developers are expanding their efforts to support the patient journey more broadly. This is being achieved through enhanced methods of training, value-added packaging and longitudinal methods of engagement that span from the onset of a disease state through various life stages.
In addition to these measures, virtually every developer in the branded and generic space is formulating some variation of a digital health approach. This often times includes add-ons or accessories that are compatible with a commercially available device and provide users with device performance feedback – like when an autoinjector is ready to inject or when an injection is complete. They can also provide error correction and other means of feedback to help eliminate use errors. The SmartPilot accessory from Ypsomed (Burgdorf, Switzerland) is an example of a device providing this level of functionality.
These devices are very effective at helping to mitigate potential use error with more mature devices. These devices also feature a mobile application that can collect and curate use information for potential integration into a patient’s health plan.
There has also been convergence within the drug delivery ecosystem, where all aspects of managing a disease state are integrated through connectivity that often times require partnerships to address broader steps in the patient journey to leverage complementary technologies. A good example of this is the partnership between Abbott Diabetes and Novo Nordisk that gathers and transmits insulin dose data from Novo Nordisk connected pens directly into the Abbott Diabetes Freestyle Libre sensor-based glucose management technology to allow patients to manage their disease states more holistically. All these ecosystems are reliant upon sensor technology and mobile applications that act as the primary interface for all the component pieces. Similar to the add-ons scenario, these ecosystems are addressing known workflows and device component constraints and are beginning to be developed into the devices themselves which will mark the next phase of electronics and connectivity in drug delivery devices./p>
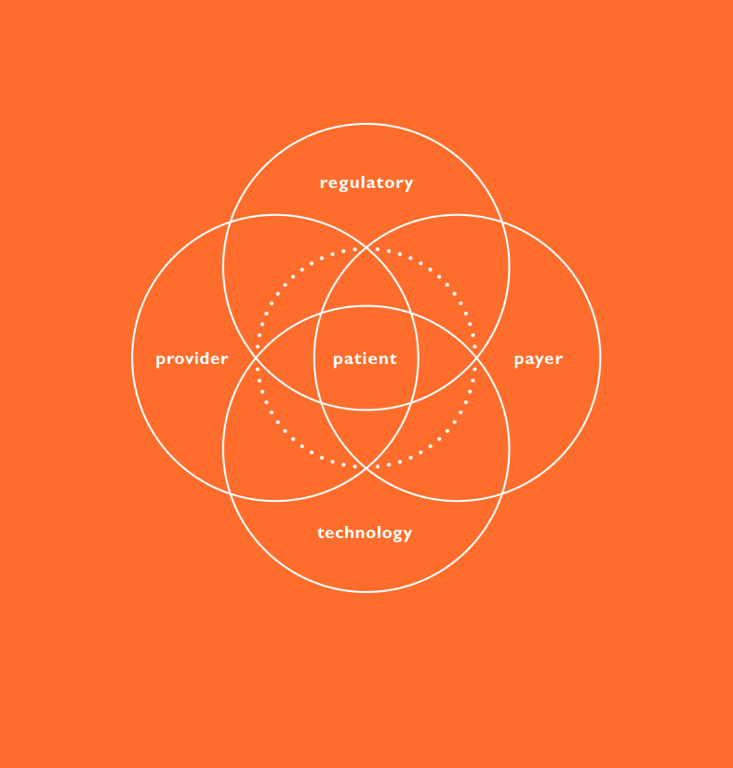
The patient journey as the foundation for integrating connectivity
Achieving success will require establishing a broad baseline of patient needs. A critical tool in helping developers formulate a user-driven strategy is a thorough mapping of the patient journey – to develop an intimate understanding of foundational user needs so that the entire journey is considered at the earliest stages of development.
A comprehensive understanding of the user enables the team to determine how these needs can be most effectively addressed, whether through the device, a software capability or with an interplay between the two augmented by other assets. This approach helps ensure developed technologies are not only meeting actual market needs; they’re doing so in an optimal way – eliminating potential redundancies and conflicts that are inevitable when developing device and software in isolation. Understanding, characterising and prioritising user needs is best achieved through applied ethnography which consists of a combination of interviews and observation within the context of use. This method can yield a deep, longitudinal understanding of the patient journey from diagnosis to treatment selection, onboarding and ongoing use. With an emphasis on system touchpoints like education/training materials, secondary packaging and device interaction, the patient journey can then be leveraged as a critical early-stage decision-making tool.
Our team of design research utilizes a method called applied ethnography to achieve this goal. This relies on interviews and in-context observations of practices, processes and experiences within the patient’s home or use environment. Potential use cases are looked at broadly beyond the administration event or complying with instructions for use. This starts from when a patient is diagnosed, to receiving their device, through the entire process of preparing, administering, and disposal and the times in between treatment to understand how the process changes over time and how frequency of administration may impact the patient experience. It is equally important to gain an understanding of the experience of healthcare professionals to consider relevant settings in clinical environments. This is important in applications where care is being provided in both in home and clinical environments as well as a migration of care, such as an oncology ward, with significant support systems to an environment of self-administration where clinical personnel are not present and the burden of support falls to a family member or caregiver.

Like drug discovery, this type of research should be started as early as possible in the development process – user needs are the cornerstone in defining the path forward. While some believe considerations surrounding the patient and device should be suspended until drug attributes are clearly defined, this approach can lead to decision making on device development or selection that can take a developer too far down a development path to make meaningful changes.
While usability testing activities are well understood when it comes to device selection and are critical at this stage, discovery research is a fundamentally different type of activity that seeks to define and understand user needs rather than help ensure they are met. User needs should be characterised as soon as there is a known target patient population – to most effectively drive the development of solutions covering the device, connectivity, mobile applications, data analytics and enhanced training that address the whole of the patient journey. The outputs from this effort include patient journey maps, clinical process maps, an understanding of prioritized user needs and values, and pain points that can be leveraged toward improving the patient and provider experience.
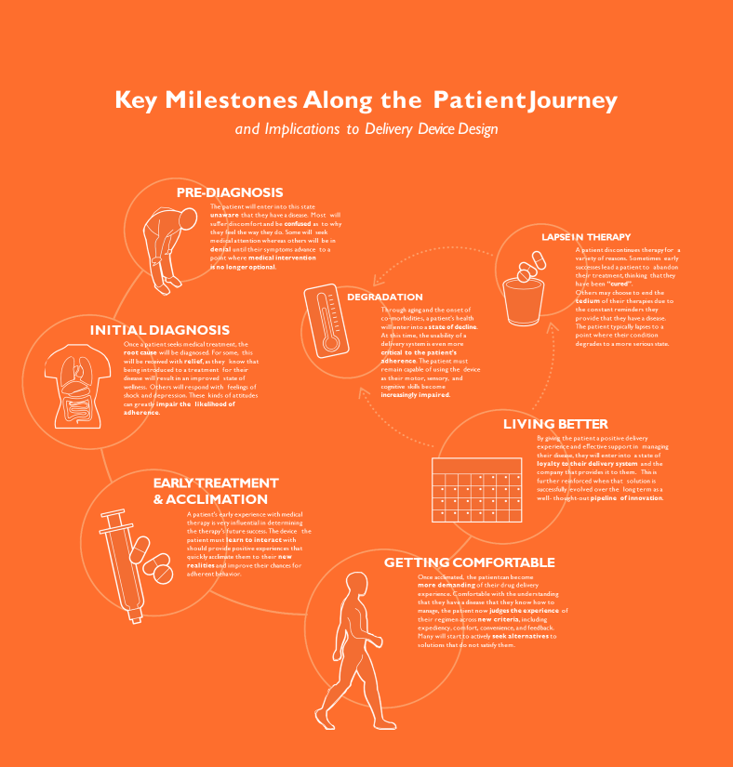
Optimizing the User Experience
Ultimately, this information can be synthesised into a development framework. This consists of mapping identified needs with corresponding device and supporting element opportunities and capabilities. The road map can then be used to guide early mapping of the ideal envisioned patient and healthcare professional journey, and the process of exploring solutions that best meet the needs at each phase of the journey while enabling the team to consider solutions more holistically. This is particularly important when determining where the integration of connectivity that can meaningfully support the experience. The road map also becomes the foundation for later requirements definition for the device, the mobile application and any other clinical interface required, ensuring that requirements stem from user needs, rather than known technical capabilities and limitations. This helps developers ensure that all elements of a device strategy for a new drug product effectively address user needs optimally and holistically.
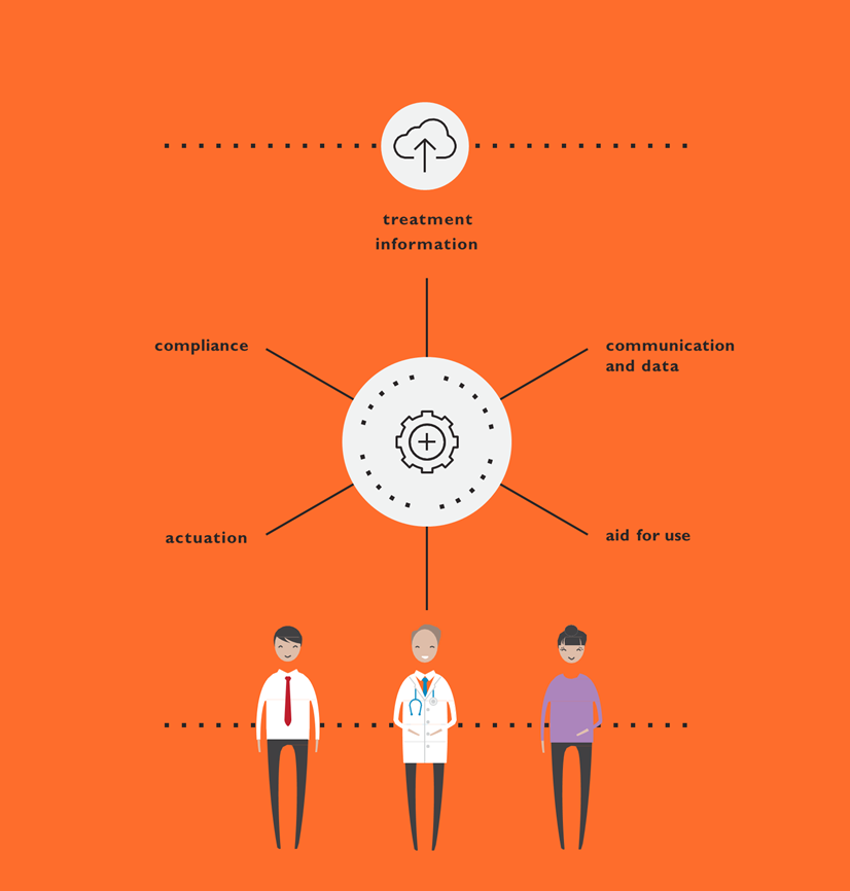
Our Insight Innovation Center can utilize this foundation to develop all aspects of device aspects from product design to particularly mobile application user interface development through an iterative process of design and evaluation sprints that integrate touch points with patients and clinical stakeholders to ensure solutions optimizing all aspects of the experience and to provide customers with robust assets for implementation by software development teams. Beyond mobile applications we can address needs holistically by addressing by packaging, both paper and digital instructions for use, and training. This approach of ‘surrounding’ device ensures that the entire experience is assembled by design and not in a piecemeal approach. Assets such as the mobile application can device connectivity can be utilized in clinical trials as well to monitor patient experience and provide critical feedback that can be incorporated into the system prior to commercial launch.
Application on a real case: Symbioze® , a smart and sustainable on-body injector
Targeting mid to long-term medication in therapeutic areas such as oncology or immunology, Symbioze® drug delivery system is a connected and reusable on-body injector suitable for high volume injections. The use of such devices is more than likely to expand as many drugs in these fields are transferred from intravenous to subcutaneous delivery, which makes the disease burden even more cumbersome on the patients’ shoulders. In order to alleviate part of this burden, the focus has been made, when designing the product, on an automated and safe injection process totally invisible for the user as well as a secured administration of the prescribed drug. Therefore, combining electronics and “proximity card” technology is Nemera’s choice to address these issues. In the same way, a Bluetooth connectivity feature has been built-in to allow data recording and sharing, notifications, reminders…etc. Although such feature is common and well-accepted in the Diabetes care, it remains innovative in cancer treatment and inflammatory pathologies.

And to get back to an example already mentioned above, oncology patients need, on top of a seamless device, to successfully achieve treatment adherence outside the hospital setting, as well as to keep a link with remote healthcare professionals. The solution that Symbioze®, the on-body injector provides can be easily integrated into their patient journey.
In order to fulfill these requirements, Nemera has chosen to entrust the German company Zollner. This new alliance will enable Nemera to offer enhanced connected drug delivery device solutions to customers and patients. As a partner-of-choice, Zollner will support the design, development and manufacturing of electronic drug delivery systems for both, Nemera’s proprietary and customer owned products.
Benefits of partnering with an integrated product and service provider
Successful navigation of these challenges often times requires a partner with a broad set of capabilities, products, and services. Nemera’s integrated development, consulting, and manufacturing services allows customers to achieve the outcome of a successful regulatory submission and commercial launch of safe, effective, and differentiated combination products with a single partner applying an agile process across the device and combination product value chain. Nemera can support every device strategy a customer may be considering from organic development to utilization of our IP platforms as well as a wide range of services to support our customer’s journey to a combination product. This is linked to our global manufacturing footprint. Ultimately, the benefits of this approach will drive:
- Patient Centricity and Engagement
The needs to patients and clinical stakeholders are centered from the onset of the program to develop a custom patient engagement strategy that is consistently considered throughout the process to maximize effectiveness when launched into the market to drive loyalty from stakeholders. - Innovation Across the Journey
Nemera’s world class design, development, and consulting capability ensures the deployment of innovative development strategies as unique as our customer’s applications for both our IP products and custom development services regardless of point of engagement. - Reduction of Risk and Increased Speed of Market Access
A single partner working across the journey limits transitions between suppliers and ensures consistent execution of strategy. Nemera also provides a wealth of options for developing and realizing a device to accelerate time to registration and market from small series to large scale manufacturing and interim supply required in between. When combined with our service offering this can significantly reduce timelines
To read the full article : HERE
About Nemera
As a world-leading drug delivery device solutions provider, our purpose of putting patients first enables us to design and manufacture devices that maximize treatment efficacy.
We are a holistic partner and help our customers succeed in the sprint to market of their combination products. From early device strategy to state-of-the-art manufacturing, we’re committed to the highest quality standards.
Agile and open-minded, we work with our customers as colleagues. Together, we go the extra mile to fulfil our mission.