NOVELIA®: A CLEAR VISION FOR EYE CARE FOR PATIENTS GLOBALLY
17/03/2023

A SAFE PRESERVATIVE-FREE SOLUTION
The majority of eye drops today contain preservatives to maintain eye drop formulation’s sterility. The most used preservative is Benzalkonium Chloride (BAK), which has been known to damage the cornea over long term use. Despite consistent data confirming its potential toxic effects, especially for chronic use, for example for glaucoma and dry eye disease, BAK is still used as the main preservative in eye drops. [1]
There have been a lot of efforts to improve the primary Container Closure System (CCS) of ophthalmic products. Multidose bottles have been improved to multidose preservative-free (MDPF) bottles. As science and technology evolves, there is a greater need to further understand the complex organ that we all cherish and would like to ‘preserve’ [2], and ensure the patient is put first.
To prevent the entry of bacteria into the bottle and/or to filter air, more than half of bottles designed for multidose preservative-free eye drops on the market rely on a filtering system; 0.22 μm sterile mesh filters being the industry standard. Significant research has been carried out that challenge their effectiveness [3]. Due to their porous structure, bacterial filters do not provide a continuous barrier to contamination.
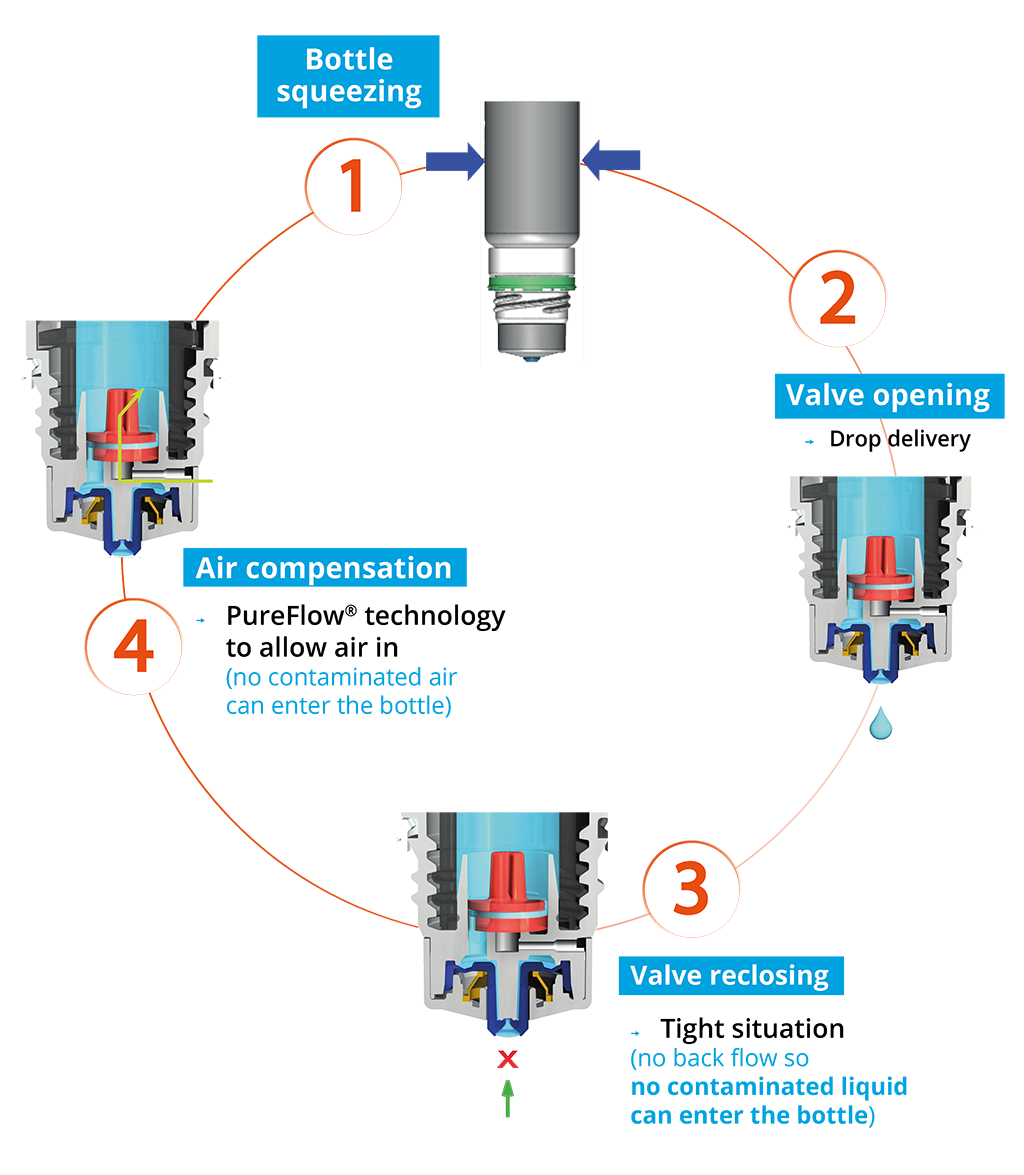
Nemera’s alternative to the use of sterile filters for multidose eye droppers for preservative free formulations is a non-return valve system used in conjunction with a silicone membrane to process the returning air. The non-return valve ensures that no contaminated liquid can be re-introduced to the container after the drop has been dispensed, completely removing the need to filter the liquid. The intake of air into the dispenser takes place via a separate venting system with a silicone membrane called the PureFlow™. The silicone membrane is a solid, non-porous (unlike bacterial filters) material. It is homogenous and does not contain any holes therefore its characteristics can be precisely engineered.
(Novelia® has) the largest amount of published information regarding the safety and sterility of these (MDPF packages … and is able to withstand both the likely microbial challenges in real-world scenarios, as well as more significant and severe challenges which could occur.
Campolo A, Crary M, Shannon P, 2022 [4]
NOVELIA®: GIVING BACK CONTROL TO PATIENTS
In 2020, a study was conducted at Nemera’s R&D department, Insight Innovation Centre, to understand these “challenges in real-world scenarios” encountered by dry eye and glaucoma patients with their current medication [5]. A total of 16 participants were included in the study. This group included patients under treatment, ophthalmologists and nurse practitioners forming the Health Care Provider (HCP) sample. The participants were asked to briefly discuss their background and experience as it related to managing their/ their patients’ eye condition including symptoms, current therapies, and treatment practices as well as relevant challenges or frustrations.
Patients commonly cited lack of control as being a key challenge relating to eye drop administration. Controlling the number of drops that are delivered was the most cited complaint from both dry eye and glaucoma patients. Patients noted more than one drop may come out at a time or even leaked a bit before being squeezed. This becomes even more problematic when the bottle is almost empty, as patients must squeeze harder to get the drop(s) out and feel they have even less control.
While a minor annoyance to dry eye patients, this is more impactful for glaucoma patients who are unsure if they’ve gotten the proper dose and/ or are concerned about their expensive medication running out prematurely. Nearly nine out of ten glaucoma patients are unable to instil eye drops correctly and therefore an easy-to-use system that is appreciated by patients could contribute to improving their compliance to a treatment. [6]
Patients and HCPs alike desire a bottle that allows users to control the number of drops delivered, consistently delivering a single drop with each actuation.
Novelia®’s PureFlow™ Technology not only serves as a venting system but also controls the medication flow. [Fig 1]. Nemera have adapted the flow-control within Novelia® that avoids multiple drop delivery into the eye and ensures that only one calibrated drop is dispensed at a time. Nemera offers three different PureFlow™ versions, each tailored to formulations of differing viscosities, from highly liquid to highly viscous. In addition, five different valve sizes are available, each one delivering a different calibrated drop size. This allows Nemera’s team to customise the drop size depending on specific product requirements. This improved control leads to increased patient confidence (of accurate dosing), reduced frustration and medication waste.
‘We Put Patients First’ is not just a motto used by Nemera, but an attitude firmly ingrained in the company’s culture. The earlier Nemera can include patients in the development phase to assess their behaviour vs. new design, the better. Patients’ needs and constraints are generally identified when defining the product design brief and are included in Nemera’s quality process and documents such as product specification, design & user FMEA.
EASE OF USE: THE TOPIC ‘IN HAND’
There is statistically significant variability in the force required to squeeze a drop from common glaucoma medications, and a representative sampling of clinic patients suggests that many likely struggle with the force requirements of several bottle designs [7].
In 2015, Nemera had user tests carried out by an independent company. The panel of patients interviewed fell in to two main criteria: demographics (age, gender) and type of eye condition (glaucoma, dry eye, etc.). These patients were interviewed in their own homes in both the US and the UK. These tests concluded that 76% of patients interviewed preferred Novelia® over other similar devices on the market [8].
Contributing factors to Novelia® preference included the intuitiveness of the screw-on cap and the associated reassurance and the squeeze force required towards the end-of-life the product. Novelia® required only 6% more pressure to squeeze the bottle from the beginning to the end of the treatment, compared to 35% for the other MDPFs. Patients, even those with dexterity issues and tremors/ shaking, must be able to effectively handle/ manipulate the delivery system and administer a drop.
I have arthritis in my hands and all the other brands are so hard to nearly not working at all to squeeze out even one drop. This dispenser works with gentle pressure every time. I am so glad to have found them,
verified Amazon US customer review, November 2022 (Systane™ Hydration PF) [9].

SUSTAINABILITY CRITERIA FOR MULTI-DOSE EYE-DROPPER
Patients also report concerns regarding unit doses. These include cost (as more packaging and eye drop solution per dose is required), waste, and convenience, as it is easier to store a multi-use bottle in a preferred location than to ensure the patient has the correct number of unit dose pipettes with them every day [10].Difficult handling has also been pointed out regarding (unit doses) and their use by older patients and with inappropriate finger manipulation could be associated with an increased risk of contamination [11]. Due in part to the rapidly aging population, the number of people having glaucoma worldwide is expected to increase to over 111 million by 2040 [12].
A comparison analysis was conducted by Nemera comparing Novelia® multidose eyedropper for preservative-free formulations with unit dose packaging for a glaucoma type regimen (one drop per eye twice per day) over a one-month treatment. The results conveyed that with Novelia® there is eight times less plastic used, 25 times less drug waste and nine times less energy needed for transportation compared to a unit dose [13].
BRINGING NOVELIA® TO PATIENTS IN CHINA
In 2022, Novelia® was successfully published on the Centre for Drug Evaluation (CDE) platform in China [14]. [Fig 3]. The CDE performs regulatory evaluation of new drugs and medical devices to assess if they can be brought to the Chinese market. Novelia®’s publication on the CDE platform ensures that Nemera’s delivery system can be referenced through the Drug Master File (DMF) number in Drug Product Applications in China. With this first milestone achieved, Nemera customers can now submit their documentation for the evaluation of their drug products with Novelia® delivery system.
Today, Novelia® has more than 250 references on the market for prescription and over-the-counter products in over 55 countries across Europe, Latin America, North America, Oceania, Middle East, and Asia Pacific.
A full range of bottles is available in low density polyethylene (LDPE), 5 mL, 7.5 mL, 11 mL, and 15 mL [Fig 4]. Novelia® has been validated using both gamma and ethylene oxide (EtO) sterilisation. Offering two options for sterilisation allows Nemera to better meet customers’ compatibility needs. Nemera can also develop additional coloured Novelia® caps for specific demands. Finally, Nemera have developed two additional cap versions as part of the Novelia® offering to combat challenging formulations, thus expanding the scope of ophthalmic treatments served by the MDPF.
NOVELIA® PRODUCTION CAPACITY EXTENSION “ACROSS THE POND”
To serve customers in supporting patient needs, Nemera is extending once again its manufacturing capabilities, this time in the US and in doing so has doubled its capacity to produce Novelia® multidose eyedropper for preservative-free formulations. [Fig 5]. Boasting over nine thousand square feet, the new ISO 7 Cleanroom, will welcome high-speed assembly lines and numerous injection moulds and injection moulding machines. In addition, this production capacity expansion in the US has created over 35 new direct and indirect positions at the Nemera Buffalo Grove plant.

A HOLISTIC APPROACH TO SUPPORT CUSTOMERS IN HELPING PATIENTS
Nemera offers a range of laboratory services for Novelia®, including testing of customer’s bulk formulation. This testing comprises usage simulation over a two-week period, drop size analysis (variable depending on valve diameter), flow control and squeeze force testing (beginning and near end of life). The culmination of these tests results allows Nemera to determine the best Novelia® configuration for a particular customer formulation. Nemera can recommend the most suitable PureFlow™ control, bottle type and valve size to achieve the desired drop calibration.
Nemera’s regulatory team is on-hand to support customers with their submission filing, providing guidance on supportive documents for registration. Nemera can also assist customers in finding the right ready-to-go dossier available for private labelling certain molecules with the Novelia® delivery system. Nemera has a substantial list of partners, formulation licensors and fillers, all working in collaboration to bring to customers a finished drug device combination product with Novelia®.
While it’s important that containers be user friendly, it has been found that educational resources instructing patients to apply their eye drops correctly mitigates many issues with unintentional noncompliance [15]. Nemera can support customers with product market launch, for example, in educating sales teams and HCPs on the delivery device, dedicated trainings, and materials to assist in promotional material creation. Customisable patient guidance videos are also available in several languages to increase patient compliance around the world.
- Gupta R et al. “Evaluating eyedrop instillation technique in glaucoma patients”. J Glaucoma, (2012), Vol 21(3), pp 189–192.
- Seshadri N., Uday B. Ophthalmic Product Development, From Bench to Bedside, Springer Cham (2021)
- Hasegawa H, Naganuma K, Nakagawa Y, Matsuyama T, Membrane filter (pore size, 0.22-0.45 μm; thickness, 150 μm) passing-through activity of Pseudomonas aeruginosa and other bacterial species with indigenous infiltration ability, FEMS Microbiology Letters 223 (2003) 41-46.
- Campolo A, Crary M, Shannon P, A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops. Biomed J Sci & Tech Res 45 (1) -(2022). BJSTR.MS.ID.007130.
- “User study performed by Insight Innovation Centre”. ICH report, Chicago, USA (2020).
- Gupta R et al. “Evaluating eyedrop instillation technique in glaucoma patients”. J Glaucoma, (2012), Vol 21(3), pp 189–192.
- Moore DB, Hammer JD, Akhtari R, Beck J, Sanders S, Kryscio RJ. Squeeze Me if You Can: Variability in Force Requirements to Extract a Drop from Common Glaucoma Bottles. J Glaucoma. (2016) Sep;25(9):780-4. doi: 10.1097/IJG.0000000000000506. PMID: 27552516; PMCID: PMC5001908.
- “User study performed for Nemera by GfK to understand the Novelia® market opportunities versus competitors (Aptar OSD system)”. GfK report, Paris, France, (2015).
- Products using the Novelia® device rate consistently high amongst patients, with an average star rating of 4.7/5 (across +4000 verified customer reviews) Amazon US customer reviews January (2023) (Systane™ Hydration PF) https://www.amazon.com/product-reviews/B0857XD9V6
- Campolo A, Crary M, Shannon P A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops. Biomed J Sci & Tech Res 45 (1) -(2022). BJSTR.MS.ID.007130.
- Bagnis A, Papadia M, Scotto R, Traverso CE. Antiglaucoma drugs: The role of preservative-free formulations. Saudi J Ophthalmol. 2011 Oct;25(4):389-94. doi: 10.1016/j.sjopt.2011.08.004. Epub (2011) Aug 28. PMID: 23960953; PMCID: PMC3729930.
- Tham, Y., Li, X., Wong, T., Quigley, H., Aung, T. and Cheng, C., (2021). Global Prevalence of Glaucoma and Projections of Glaucoma Burden Through 2040.
- “Comparison analysis conducted by Nemera comparing Novelia® multidose eyedropper for preservative-free formulations with unit dose packaging,” Nemera, La Verpillière, France, (2020).
- “Novelia®, published on CDE (Centre for Drug Evaluation) platform in China!” (12/10/2022) Nemera website: https://www.nemera.net/success-stories/novelia-published-on-cde-center-for-drug-evaluation-platform-in-china/
- Davis SA, Carpenter DM, Blalock SJ, Budenz DL, Lee C, Muir KW, Robin AL, Sleath B. A randomized controlled trial of an online educational video intervention to improve glaucoma eye drop technique. Patient Educ Couns. 2019 May;102(5):937-943. doi: 10.1016/j.pec.2018.12.019. Epub (2018) Dec 18. PMID: 30583913.
About Nemera
As a world-leading drug delivery device solutions provider, our purpose of putting patients first enables us to design and manufacture devices that maximize treatment efficacy.
We are a holistic partner and help our customers succeed in the sprint to market of their combination products. From early device strategy to state-of-the-art manufacturing, we’re committed to the highest quality standards.
Agile and open-minded, we work with our customers as colleagues. Together, we go the extra mile to fulfil our mission.


