A unique approach to achieve bioequivalence requirements
Our Device Equivalence Program will give you a high confidence level on results of the final product registration thanks to this preliminary bioequivalence study.
Its objective is to propose a delivery system with comparable performance to the branded device in terms of design, patient usage and performance characteristics.
Our Device Equivalence Program is based on FDA requirements:
- Specific program to meet requirements for in-vitro bioequivalence
- Customized testing methodologies with state-of-the-art lab equipment
- Advanced statistical analysis
We can perform in-vitro bioequivalence study and tests with your formulations in our laboratory and support your registration process:
- In-vitro bioequivalence testing
- Different statistical calculations depending on regional needs
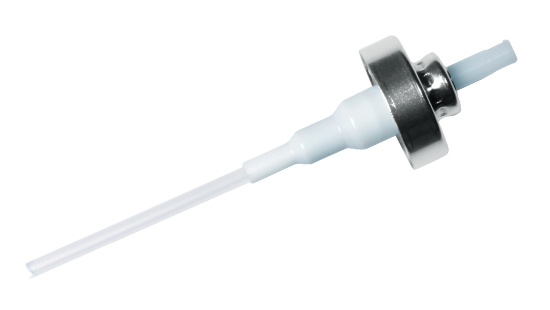
We also have developed off-the-shelf bioequivalent devices for some key nasal drug products.
- Off-the-shelf devices with IVBE requirements on priming/ re-priming, dose delivery and spray characteristics
- Several pump/ actuator combinations developed
- “Fast-track” program meeting FDA IVBE requirements
- Based on track-record SP270+ platform and customized actuators
- In vitro Bioequivalence testing with state-of-the-art lab equipment, from screening tests to registration purposes
- Dedicated program & teams to answer a comprehensive clients’ support, including regulatory and statistics